667463-62-9
Product Name:
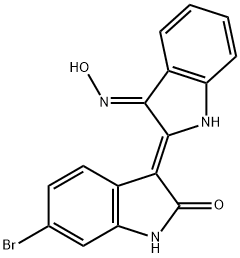
BIO
Formula:
C16H10BrN3O2
Synonyms:
BIO, (2ʹ Z,3ʹ E)-6-Bromoindirubin-3ʹ-oxime;BIO, (2ʹZ,3ʹE)-6-Bromoindirubin-3ʹ-oxime;GSK-3 Inhibitor IX - CAS 667463-62-9 - Calbiochem;InSolution GSK-3 Inhibitor IX - CAS 667463-62-9 - Calbiochem
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 356.17 g/mol |
|---|---|
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 354.99564 g/mol |
| Monoisotopic Mass | 354.99564 g/mol |
| Topological Polar Surface Area | 81.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 438 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
6-bromoindirubin-3'-oxime is a member of the class of biindoles that is indirubin substituted at position 6 by a bromo group and in which the keto group at position 3' has undergone condensation with hydroxylamine to form the corresponding oxime. It has a role as an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and an EC 2.7.11.26 (tau-protein kinase) inhibitor. It is a ketoxime, an organobromine compound, a member of oxindoles and a biindole.