66357-59-3
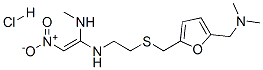
Product Name:
Ranitidine Hydrochloride
Formula:
C13H23ClN4O3S
Synonyms:
N, N Dimethyl-5-[2-(1-methylamine-2-nitrovinyl)-ethylthiomethyl]furfurylamine hydrochloride;Ranitidine hydrochloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Chemical Classes | Other Uses -> Pharmaceuticals |
|---|
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 350.87 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 350.1179395 g/mol |
| Monoisotopic Mass | 350.1179395 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 347 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ranitidine Hydrochloride is a member of the class of histamine H2-receptor antagonists with antacid activity. Ranitidine is a competitive and reversible inhibitor of the action of histamine, released by enterochromaffin-like (ECL) cells, at the histamine H2-receptors on parietal cells in the stomach, thereby inhibiting the normal and meal-stimulated secretion of stomach acid. In addition, other substances that promote acid secretion have a reduced effect on parietal cells when the H2 receptors are blocked.
