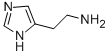CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | NEEDLES FROM CHLOROFORM |
| Boiling Point | 209-210 °C @ 18 MM HG |
| Melting Point | 139 °C (phosphate salt) |
| Solubility | 14.1 [ug/mL] (The mean of the results at pH 7.4) |
| LogP | -0.70 |
| Stability/Shelf Life | STABLE IN AIR BUT IS AFFECTED BY LIGHT /PHOSPHATE/ |
| Dissociation Constants | 9.756 |
| Collision Cross Section | 120 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Kovats Retention Index | 1500 1497 |
| Other Experimental Properties | DELIQUESCENT |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
COMPUTED DESCRIPTORS
| Molecular Weight | 111.15 g/mol |
|---|---|
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 111.079647300 g/mol |
| Monoisotopic Mass | 111.079647300 g/mol |
| Topological Polar Surface Area | 54.7 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 64.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Histamine is a member of the class of imidazoles that is 1H-imidazole substituted at position C-4 by a 2-aminoethyl group. It has a role as a human metabolite, a mouse metabolite and a neurotransmitter. It is an aralkylamino compound and a member of imidazoles. It is a conjugate base of a histaminium.