SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 170.08 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 169.967113 g/mol |
| Monoisotopic Mass | 169.967113 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
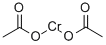
Chromium(II) acetate is a coordination compound of chromium. It is used the polymer industry, to dehalogenate organic compounds such as α-bromoketones and chlorohydrins, and as an oxygen scrubber. Chromium is a chemical element which has the symbol Cr and atomic number 24. It is found naturally occuring in rocks, animals, plants, and soil, and is usually mined as chromite ore. Chromium is most toxic in its +6 oxidation state (chromium(VI)) due to its greater ability to enter cells and higher redox potential. Trivalent chromium (chromium(III)) however, is biologically necessary for sugar and lipid metabolism in humans. (L17, L518)