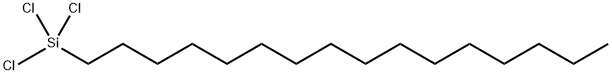
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Hexadecyltrichlorosilane is a colorless to yellow liquid with a pungent odor. Material will burn though it may require some effort to ignite. It is decomposed by moisture or water to hydrochloric acid with evolution of heat. It is corrosive to metals and tissue. |
|---|---|
| Color/Form | Colorless to yellow liquid |
| Boiling Point | 269 °C |
| Flash Point | 295 °F (146 °C) (Closed cup) |
| Density | 0.996 at 25 °C/25 °C |
| Stability/Shelf Life | Evolves hydrogen chloride in the presence of moisture. |
| Decomposition | The silanes decomp at elevated temp to liberate hydrogen and deposit a high purity silicon, which leads to some of the principal uses of silanes. /Silanes/ |
| Corrosivity | Corrosive |
| Refractive Index | Index of refraction = 1.4568 at 25 °C/D |
| Other Experimental Properties | The reaction of organosilanes with halogens and halogen cmpd usually proceeds in good yield through cleavage of the Si--H bond and formation of the silicon-halogen bond. Reaction with fluorine, however, does not proceed satisfactorily because of cleavage of not only the Si--H but also C--Si and C--H bonds. Direct halogenation with chlorination, bromine, and iodine proceeds smoothly, however. /Organosilanes/ |
| Chemical Classes | Toxic Gases & Vapors -> Chlorosilanes |
COMPUTED DESCRIPTORS
| Molecular Weight | 359.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 14 |
| Exact Mass | 358.141711 g/mol |
| Monoisotopic Mass | 358.141711 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 193 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Hexadecyltrichlorosilane is a colorless to yellow liquid with a pungent odor. Material will burn though it may require some effort to ignite. It is decomposed by moisture or water to hydrochloric acid with evolution of heat. It is corrosive to metals and tissue.