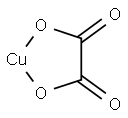
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cupric oxalate is an odorless bluish-white solid. Denser than water and insoluble in water. Hence sinks in water. Used as a catalysts for organic reactions. |
|---|---|
| Color/Form | Blue-white powder |
| Solubility | Solubility: 0.00253 g/100 ml water @ 25 °C /Hemihydrate/ |
| Density | greater than 1 at 68 °F (USCG, 1999) |
| Decomposition | Dangerous; when heated to decomposition they emit toxic and irritating fumes. /Oxalates/ |
| Other Experimental Properties | Loses any hydrated water by 200 °C; decomp in air @ 310 °C to cupric oxide. |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P321:Specific treatment (see … on this label). |
COMPUTED DESCRIPTORS
| Molecular Weight | 151.56 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 150.909256 g/mol |
| Monoisotopic Mass | 150.909256 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 60.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cupric oxalate is an odorless bluish-white solid. Denser than water and insoluble in water. Hence sinks in water. Used as a catalysts for organic reactions.