5536-17-4
Product Name:
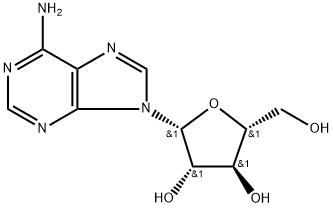
Vidarabine
Formula:
C10H13N5O4
Synonyms:
9-β-D -Arabinofuranosyladenine;Ara-A;Vidarabine
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Vidarabine is a white to off-white crystalline powder. (NTP, 1992) |
|---|---|
| Color/Form | Crystals from water |
| Melting Point | 500 to 518 °F (NTP, 1992) |
| Solubility | >40.1 [ug/mL] (The mean of the results at pH 7.4) |
| LogP | -2.115 |
| Stability/Shelf Life | Vidarabine ophthalmic ointment should be stored at a temperature less than 40 °C, preferably between 15-30 °C; freezing should be avoided. |
| Optical Rotation | Specific optical rotation: -5 deg at 27 °C/D (concentration by volume= 0.25 g in 100 ml water). |
| Decomposition | When heated to decomposition it emits very toxic fumes of /nitrogen oxides/. |
| Collision Cross Section | 156.4 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 267.24 g/mol |
|---|---|
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 267.09675391 g/mol |
| Monoisotopic Mass | 267.09675391 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 335 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Vidarabine is a white to off-white crystalline powder. (NTP, 1992)
