53885-35-1
Product Name:
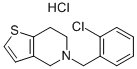
Ticlopidine hydrochloride
Formula:
C14H14ClNS.ClH
Synonyms:
5-(o-Chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | 2.9 [ug/mL] (The mean of the results at pH 7.4) |
|---|---|
| Collision Cross Section | 154.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 300.2 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 299.0302260 g/mol |
| Monoisotopic Mass | 299.0302260 g/mol |
| Topological Polar Surface Area | 31.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 261 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ticlopidine Hydrochloride is the hydrochloride salt form of ticlopidine, a thienopyridine derivative with anticoagulant property. Ticlopidine hydrochloride irreversibly inhibits adenosine-diphosphate (ADP)-induced platelet-fibrinogen binding by binding to the glycoprotein (GP) IIb/IIIA complex, one of the two purinergic receptors activated by ADP. Inhibition of the receptor activation causes the inhibition of adenylyl cyclase, results in decreased levels of cyclic adenosine monophosphate and thereby interferes with platelet membrane function and subsequent, platelet-platelet interaction, release of platelet granule constituents and prolongation of bleeding time.
