52806-53-8
Product Name:
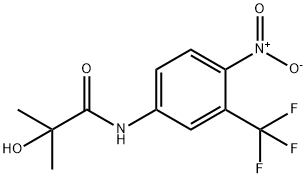
HYDROXYFLUTAMIDE
Formula:
C11H11F3N2O4
Synonyms:
2-Hydroxy-2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]-propanamide;2-Hydroxyflutamide;a,a,a-Trifluoro-2-methyl-4′-nitro-m-lactotoluidide;Hydroxyniphtholide;Sch 16423
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 292.21 g/mol |
|---|---|
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 292.06709132 g/mol |
| Monoisotopic Mass | 292.06709132 g/mol |
| Topological Polar Surface Area | 95.2 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 392 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
2-hydroxyflutamide is a metabolite of flutamide. Flutamide is an oral nonsteroidal antiandrogen drug primarily used to treat prostate cancer. It competes with testosterone and its powerful metabolite, dihydrotestosterone (DHT) for binding to androgen receptors in the prostate gland. By doing so, it prevents them from stimulating the prostate cancer cells to grow. Flutamide has been largely replaced by a newer member of this class, bicalutamide, due to a better side-effect profile. (Wikipedia)
