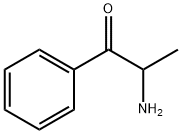
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Pale yellow leaf from petroleum ether |
|---|---|
| Boiling Point | BP: 93 °C at 0.8 mm Hg |
| Melting Point | 46.5 °C |
| Solubility | Very soluble in ether, ethanol |
| Stability/Shelf Life | A primary concern with the forensic analysis of the khat plant (Catha edulis) has been the need to preserve the principle psychoactive component, cathinone, which converts to the less-active substance, cathine, after harvesting. The loss of cathinone has serious legal implications since it is a Schedule I controlled substance under federal regulations in the United States, while cathine is Schedule IV. A common misconception is that cathinone is highly unstable once the plant is harvested, and may be undetectable upon drying and prolonged storage. However, drying the plant material will preserve cathinone. Numerous seizures of a dried form of khat (referred to as "graba" in the United States) have been made in recent years, suggesting that drying the plant material is a viable approach to preserve khat evidence for both storage and reanalysis. A qualitative and quantitative study of the composition of khat samples seized as dried plant material has found the khat alkaloids to be relatively stable for a monitored period of 3 years, and cathinone has remained identifiable while stored at room temperature for over 10 years. Studies of green khat (received moist) have also determined that drying the moist leaves at either room temperature or by the application of heat are suitable methods to preserve cathinone in the dried material. These findings demonstrate that cathinone persists in dried khat for a time frame of several years, and simple drying techniques are an effective means to preserve seized khat evidence for long-term storage. |
| Decomposition | Hazardous decomposition products formed under fire conditions. - Carbon oxides, nitrogen oxides (NOx), Hydrogen chloride gas. /S(-)-Cathinone hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 149.19 g/mol |
|---|---|
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 149.084063974 g/mol |
| Monoisotopic Mass | 149.084063974 g/mol |
| Topological Polar Surface Area | 43.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 139 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
2-aminopropiophenone is propiophenone substituted at the beta-carbon by an amino group. It is a primary amino compound and an aromatic ketone. It is functionally related to a propiophenone.