522-17-8
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 194.2 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 394.4 g/mol |
|---|---|
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 394.14163842 g/mol |
| Monoisotopic Mass | 394.14163842 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 674 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
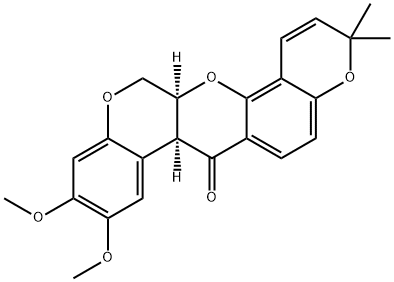
Deguelin is a rotenone that is 13,13a-dihydro-3H-chromeno[3,4-b]pyrano[2,3-h]chromen-7(7aH)-one substituted by methoxy groups at positions 9 and 10, and by two methyl groups at position 3 (the 7aS,13aS-stereoisomer). It exists in abundant quantities in the bark, roots, and leaves of the Leguminosae family of plants and reported to exert anti-tumour effects in various cancers. It has a role as an apoptosis inducer, an antineoplastic agent, a plant metabolite, an angiogenesis inhibitor, an antiviral agent, a mitochondrial NADH:ubiquinone reductase inhibitor, an anti-inflammatory agent and an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor. It is a member of rotenones, an aromatic ether, an organic heteropentacyclic compound and a diether.
