CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 174.5 °C |
| Collision Cross Section | 144.8 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 1980 1929 1967 1968 1980 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 204.27 g/mol |
|---|---|
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 204.126263138 g/mol |
| Monoisotopic Mass | 204.126263138 g/mol |
| Topological Polar Surface Area | 39.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 208 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
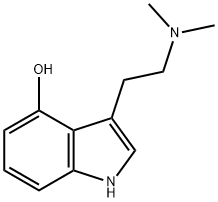
Psilocin is a tryptamine alkaloid that is N,N-dimethyltryptamine carrying an additional hydroxy substituent at position 4. A hallucinogenic alkaloid isolated in trace amounts from Psilocybe mushrooms (also known as Teonanacatl or "magic mushrooms"). It has a role as a hallucinogen, a serotonergic agonist, a fungal metabolite, a human xenobiotic metabolite and a drug metabolite. It is a tryptamine alkaloid, a tertiary amino compound, a member of hydroxyindoles and a member of phenols. It is functionally related to a N,N-dimethyltryptamine. It is a conjugate base of a psilocinium.