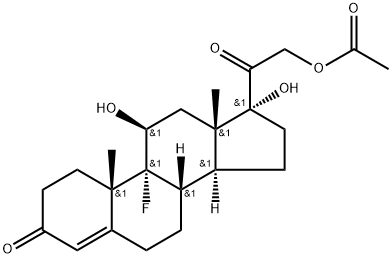
514-36-3
CHEMICAL AND PHYSICAL PROPERTIES
| Collision Cross Section | 189.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H361:Reproductive toxicity |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 422.5 g/mol |
|---|---|
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 422.21046687 g/mol |
| Monoisotopic Mass | 422.21046687 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 838 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Fludrocortisone acetate is an acetate ester resulting from the formal condensation of the primary hydroxy group of fludrocortisone with acetic acid. A synthetic corticosteroid, it has glucocorticoid actions about 10 times as potent as hydrocortisone, while its mineralocorticoid actions are over 100 times as potent. It is used in partial replacement therapy for primary and secondary adrenocortical insufficiency in Addison's disease and for the treatment of salt-losing adrenal hyperplasia. It is an 11beta-hydroxy steroid, a 3-oxo-Delta(4) steroid, a 17alpha-hydroxy steroid, an acetate ester, a mineralocorticoid, a 20-oxo steroid, a fluorinated steroid and a tertiary alpha-hydroxy ketone. It is functionally related to a fludrocortisone.