CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Orthorhombic crystals from benzene |
|---|---|
| Melting Point | 175 °C (decomposes) |
| Solubility | Insoluble in water |
| Optical Rotation | Specific optical rotation: -183 deg at 20 °C/D (chloroform) |
| Other Experimental Properties | MW: 705.90 /Ergocristine methanesulfonate/ |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 609.7 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 609.29511936 g/mol |
| Monoisotopic Mass | 609.29511936 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1270 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
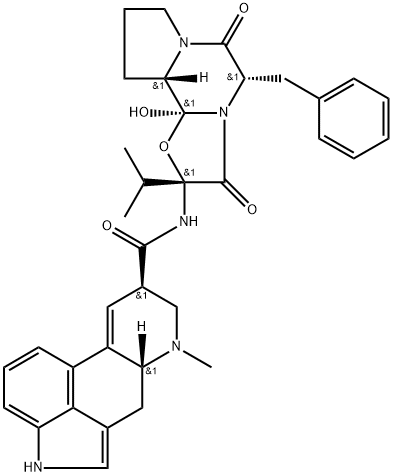
Ergocristine is ergotaman bearing benzyl, hydroxy, and isopropyl groups at the 5', 12' and 2' positions, respectively, and oxo groups at positions 3', 6', and 18. It is a natural ergot alkaloid. It derives from a hydride of an ergotaman.