CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Yellow, odorless solid; [HSDB] Technical grade is a dark red solution; [CHEMINFO] Commercially available as an aqueous solution with surfactants; [ACGIH] |
|---|---|
| Color/Form | Yellow solid |
| Odor | Odorless |
| Boiling Point | Boiling point (760 mm Hg): Decomposes at 175-180 °C (347-356 °F) |
| Melting Point | Colorless crystals; mp: 300 °C (decomposes); very soluble in water, slightly soluble in lower alcohols. Insoluble in hydrocarbons. Hydrolyzed by alkali. Inactivated by inert clays and anionic surfactants; corrosive to metal; non-volatile /Paraquat dichloride/ |
| Solubility | In water, 6.2X10+5 mg/L at 20 °C |
| Density | 1.24 |
| Vapor Pressure | 0.0000001 [mmHg] |
| LogP | log Kow = -4.22 at pH 7.4 |
| Stability/Shelf Life | Paraquat is stable in acid or neutral solutions, but is readily hydrolyzed by alkali. |
| Decomposition | When heated to decomposition it emits toxic fumes of /nitric oxides/. |
| Corrosivity | Paraquat is corrosive to metals. |
| Other Experimental Properties | Colorless crystalline solid. Stable except under alkaline conditions. No measurable vapor pressure. Dcomposes at approximately 300 °C. Sparingly soluble in lower alcohols; insoluble in hydrocarbons /Paraquat dichloride/ |
| Chemical Classes | Pesticides -> Herbicides, Bipyridyl |
COMPUTED DESCRIPTORS
| Molecular Weight | 186.25 g/mol |
|---|---|
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 1 |
| Exact Mass | 186.115698455 g/mol |
| Monoisotopic Mass | 186.115698455 g/mol |
| Topological Polar Surface Area | 7.8 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 2 |
| Complexity | 145 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
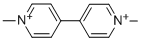
Paraquat is an organic cation that consists of 4,4'-bipyridine bearing two N-methyl substituents loctated at the 1- and 1'-positions. It has a role as a herbicide and a geroprotector. It derives from a hydride of a 4,4'-bipyridine.