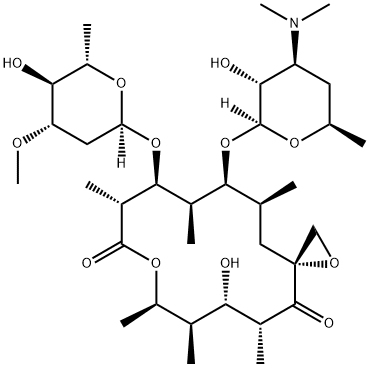
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White, amorphous powder |
|---|---|
| Solubility | Sol in dil acids. Freely sol in methanol, ethanol, butanol, acetone. Practically insol in hexane, carbon tetrachloride, dibutyl ether |
| LogP | log Kow = 1.69 |
| Dissociation Constants | 8.84 |
| Other Experimental Properties | Long needles from ethyl acetate, mp 134-135 °C. Specific optical rotation = -54 °For D (sodium) line at 25 °C (methanol). Freely sol in water. Forms various cryst hydrates /Hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 687.9 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 6 |
| Exact Mass | 687.41937638 g/mol |
| Monoisotopic Mass | 687.41937638 g/mol |
| Topological Polar Surface Area | 166 Ų |
| Heavy Atom Count | 48 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Oleandomycin is a member of oleandomycins. It is functionally related to an oleandolide. It is a conjugate base of an oleandomycin(1+).