38396-39-3
Product Name:
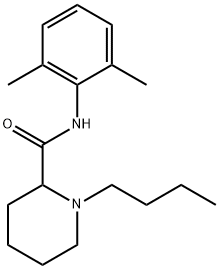
(±)-1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
Formula:
C18H28N2O
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 107-108 °C |
| Solubility | 2400 mg/L (at 25 °C) |
| LogP | 3.41 |
| Optical Rotation | Crystals from isopropanol, mp 255-257 °C. Specific optical rotation: -80.9 deg at 25 °C (c = 2 in methanol) /Bupivacaine (-) form/ |
| Dissociation Constants | 8.1 |
| Collision Cross Section | 173.1 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Kovats Retention Index | 2278 |
| Other Experimental Properties | Partition coefficient: (oleyl alcohol/water) 1565; (n-heptane/pH 7.4 buffer) 27.5 |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 288.4 g/mol |
|---|---|
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 288.220163521 g/mol |
| Monoisotopic Mass | 288.220163521 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 321 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide is a piperidinecarboxamide obtained by formal condensation of the carboxy group of N-butylpipecolic acid with the amino group of 2,6-dimethylaniline. It is a piperidinecarboxamide, an aromatic amide and a tertiary amino compound. It is a conjugate base of a 1-butyl-2-[(2,6-dimethylphenyl)carbamoyl]piperidinium.