SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 542.0 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 8 |
| Exact Mass | 541.2091968 g/mol |
| Monoisotopic Mass | 541.2091968 g/mol |
| Topological Polar Surface Area | 90.4 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 743 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
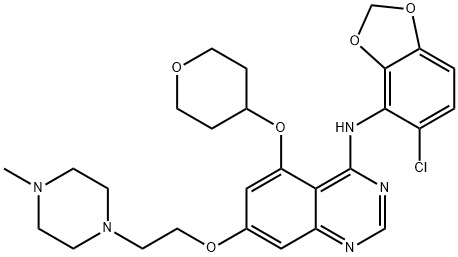
Saracatinib is a member of the class of quinazolines that is quinazoline substituted by (5-chloro-2H-1,3-benzodioxol-4-yl)amino, (oxan-4-yl)oxy and 2-(4-methylpiperazin-1-yl)ethoxy groups at positions 4, 5 and 7, respectively. It is a dual inhibitor of the tyrosine kinases c-Src and Abl (IC50 = 2.7 and 30 nM, respectively). Saracatinib was originally developed by AstraZeneca for the treatment of cancer but in 2019 it was granted orphan drug designation by the US Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (IPF), a type of lung disease that results in scarring (fibrosis) of the lungs. It has a role as an antineoplastic agent, an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, a radiosensitizing agent, an autophagy inducer, an apoptosis inducer and an anticoronaviral agent. It is a member of quinazolines, a secondary amino compound, a N-methylpiperazine, an aromatic ether, a member of oxanes, a member of benzodioxoles, an organochlorine compound and a diether.