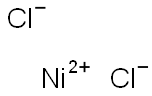CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Nickel chloride appears as a brown or green colored solid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals. |
|---|---|
| Color/Form | Yellow scales; ... golden yellow |
| Odor | None |
| Boiling Point | Sublimes @ 973 °C; deliquesces |
| Melting Point | 1001 °C |
| Solubility | Green, deliquescent crystals or crystal powder, monoclinic, structure reported to be trans-(NiCl2(H2O)4.2H2O, sol in about one part water, in alcohol /Hexahydrate/ |
| Density | 3.55 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | 1 mm Hg at 671 °C (solid) |
| Decomposition | When heated to decomp it emits very toxic fumes of /hydrogen chloride/. |
| Other Experimental Properties | Golden-yellow, sublimable in absence of air, readily absorbs NH3. /Anhydr salt/ |
| Chemical Classes | Metals -> Nickel Compounds, Inorganic |
COMPUTED DESCRIPTORS
| Molecular Weight | 129.60 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 127.873047 g/mol |
| Monoisotopic Mass | 127.873047 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Nickel chloride appears as a brown or green colored solid. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.