CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder |
|---|---|
| Color/Form | Flat needles from alcohol + ether; prisms from water |
| Odor | Odorless |
| Taste | Sweet |
| Melting Point | 221 dec °C |
| Solubility | 162000 mg/L (at 25 °C) |
| Density | 1.064 at 24 °C |
| LogP | -2.54 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Optical Rotation | SPECIFIC OPTICAL ROTATION: -80.9 DEG @ 20 °C/D (WATER, 1%); SADTLER REF NUMBER: 1905 (IR, PRISM) |
| Decomposition | When heated to decomposition it emits toxic vapors of /nitrogen oxides/. |
| Ionization Efficiency | Positive |
| Dissociation Constants | pK1 = 1.99 /carboxylic/; pK2 = 10.60 /amine/ |
| Collision Cross Section | 125.1 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Other Experimental Properties | Isoelectric point: 6.30 |
| Chemical Classes | Biological Agents -> Amino Acids and Derivatives |
COMPUTED DESCRIPTORS
| Molecular Weight | 115.13 g/mol |
|---|---|
| XLogP3 | -2.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 115.063328530 g/mol |
| Monoisotopic Mass | 115.063328530 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 103 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
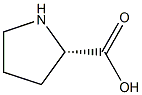
L-proline is pyrrolidine in which the pro-S hydrogen at position 2 is substituted by a carboxylic acid group. L-Proline is the only one of the twenty DNA-encoded amino acids which has a secondary amino group alpha to the carboxyl group. It is an essential component of collagen and is important for proper functioning of joints and tendons. It also helps maintain and strengthen heart muscles. It has a role as a micronutrient, a nutraceutical, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a member of compatible osmolytes. It is a glutamine family amino acid, a proteinogenic amino acid, a proline and a L-alpha-amino acid. It is a conjugate base of a L-prolinium. It is a conjugate acid of a L-prolinate. It is an enantiomer of a D-proline. It is a tautomer of a L-proline zwitterion.