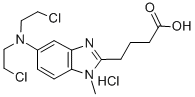
3543-75-7
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H341:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 394.7 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 9 |
| Exact Mass | 393.077760 g/mol |
| Monoisotopic Mass | 393.077760 g/mol |
| Topological Polar Surface Area | 58.4 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 380 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bendamustine Hydrochloride is the hydrochloride salt of bendamustine, a bifunctional mechlorethamine derivative with alkylator and antimetabolite activities. Bendamustine possesses three active moieties: an alkylating group; a benzimidazole ring, which may act as a purine analogue; and a butyric acid side chain. Although its exact mechanism of action is unknown this agent appears to act primarily as an alkylator. Bendamustine metabolites alkylate and crosslink macromolecules, resulting in DNA, RNA and protein synthesis inhibition, and, subsequently, apoptosis. Bendamustine may differ from other alkylators in that it may be more potent in activating p53-dependent stress pathways and inducing apoptosis; it may induce mitotic catastrophe; and it may activate a base excision DNA repair pathway rather than an alkyltransferase DNA repair mechanism. Accordingly, this agent may be more efficacious and less susceptible to drug resistance than other alkylators.