SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 438.5 g/mol |
|---|---|
| XLogP3 | 5.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 438.20423867 g/mol |
| Monoisotopic Mass | 438.20423867 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 698 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
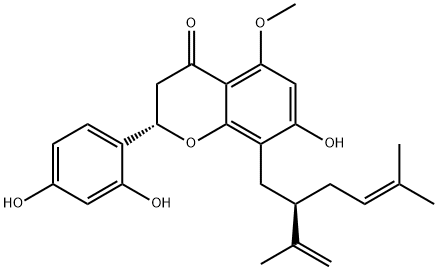
(2S)-(-)-kurarinone is a trihydroxyflavanone that is (2S)-flavanone substituted by hydroxy groups at positions 7, 2' and 4', a lavandulyl group at position 8 and a methoxy group at position 5. Isolated from the roots of Sophora flavescens, it exhibits cytotoxicity against human myeloid leukemia HL-60 cells. It has a role as a metabolite and an antineoplastic agent. It is a trihydroxyflavanone, a monomethoxyflavanone and a member of 4'-hydroxyflavanones. It is functionally related to a (2S)-flavanone.