CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Pale yellow oil |
| Boiling Point | BP: 52 °C at 0.005 mm Hg |
| Melting Point | 233-234 °C |
| Solubility | 312 mg/ml |
| LogP | 3.6 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Dissociation Constants | 8.35 |
| Collision Cross Section | 157.8 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)] |
| Other Experimental Properties | Crystals from isopropanol and absolute ethanol. MP: 233-234 °C. Bitter, anesthetizing taste. Solubility (mg/mL): water 312; alcohol 193; 0.1N HCl 333. pKa 7.9 /Bupropion hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 239.74 g/mol |
|---|---|
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 239.1076919 g/mol |
| Monoisotopic Mass | 239.1076919 g/mol |
| Topological Polar Surface Area | 29.1 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 247 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
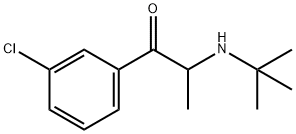
Bupropion is an aromatic ketone that is propiophenone carrying a tert-butylamino group at position 2 and a chloro substituent at position 3 on the phenyl ring. It has a role as an antidepressant, an environmental contaminant and a xenobiotic. It is a secondary amino compound, a member of monochlorobenzenes and an aromatic ketone.