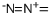| Physical Description |
Diazomethane is a yellow gas with a musty odor that is shipped as a liquid under pressure. (NIOSH, 2022) Highly toxic by inhalation. |
| Color/Form |
Yellow gas [Note: Shipped as a liquefied compressed gas] |
| Odor |
Musty odor |
| Boiling Point |
-9 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point |
-229 °F (NIOSH, 2023) |
| Flash Point |
Flammable gas |
| Solubility |
Reacts with water (NIOSH, 2023) |
| Density |
1.45 |
| Vapor Density |
1.45 (NIOSH, 2023) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure |
greater than 1 atm (NIOSH, 2023) |
| LogP |
2.00 (estimated) |
| Autoignition Temperature |
Explodes at 100 °C (212 °F) or if impurities are present, at lower temperatures. Vapor may explode at temperatures above 200 °C (392 °F). |
| Decomposition |
WHEN HEATED TO DECOMPOSITION ... EMITS HIGHLY TOXIC FUMES OF /NITROGEN OXIDES./ SRP: DIAZOMETHANE DOES NOT NEED TO DECOMPOSE TO EMIT TOXIC FUMES. |
| Ionization Potential |
9.00 eV |
| Other Experimental Properties |
Undiluted liquid and concentrated solutions may explode violently, especially if impurities are present. Gaseous diazomethane may explode on heating to 100 °C or on rough glass surfaces. Ground glass apparatus and glass stirrers with glass sleeve bearings where grinding may occur, should not be used. Alkali metals also produce explosions with diazomethane. Soluble in ether, dioxane. Such solutions decompose only slowly at low temperatures. Decomposition is more rapid if alcohols or water are present. Copper powder causes active decomposition with the evolution of nitrogen and the formation of insoluble white flakes of polymethylene (CH2)x. Solid calcium chloride or boiling stones have the same effect. This phenomenon appears to occur always during the action of diazomethane on solid substances. |
| Chemical Classes |
Nitrogen Compounds -> Other Nitrogen Compounds |