303-81-1
Product Name:
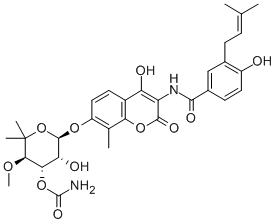
Novobiocin
Formula:
C31H36N2O11
Synonyms:
Benzamide, N-[7-[[3-O-(aminocarbonyl)-6-deoxy-5-C-methyl-4-O-methyl-?-l-lyxohexopyranosyl]oxy]-4-hydroxy-8-methyl-2-oxo-2H-1-benzopyran-3-yl]-4-hydroxy-3-
(3-methyl-2-butenyl), monohydrate;Streptonivicin
Inquiry
(3-methyl-2-butenyl), monohydrate;Streptonivicin
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Pale yellow orthorhombic crystals from ethanol |
| Melting Point | 152-156 °C (decomposes) |
| Solubility | Insoluble |
| Density | 1.3448 g/cu cm |
| LogP | 4.1 |
| Optical Rotation | Specific optical rotation at 24 °C for D (sodium) line = -63.0 deg (c = 1 in ethanol) |
| Dissociation Constants | 4.3 |
| Other Experimental Properties | Mol wt: 634.60. Minute crystals, dec 220 °C. Specific optical rotation at 24 °C for D (sodium) line = -38 deg (c = 2.5 in 95% ethanol); Specific optical rotation at 24 °C for D (sodium) line = -33 deg (c = 2.5 in water). Freely sol in water. A 100 mg/ml soln has a pH of 7.5 and a half-life of about 30 days at 25 °C and several months at 4 °C /Monosodium salt/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 612.6 g/mol |
|---|---|
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 612.23190997 g/mol |
| Monoisotopic Mass | 612.23190997 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Novobiocin is a coumarin-derived antibiotic obtained from Streptomyces niveus. It has a role as an antibacterial agent, an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an antimicrobial agent, an Escherichia coli metabolite and a hepatoprotective agent. It is a hexoside, a monocarboxylic acid amide, a monosaccharide derivative, a hydroxycoumarin, an ether, a carbamate ester and a member of phenols. It is a conjugate acid of a novobiocin(1-).