29767-20-2
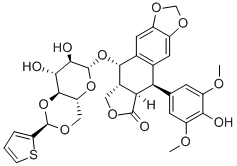
Product Name:
Teniposide
Formula:
C32H32O13S
Synonyms:
4′-Dimethyl-9-(4,6-O-2-thenyid)-epipodophyllotoxin;Tenoposide;VM-26
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Crystals from absolute ethanol |
|---|---|
| Melting Point | 242-246 °C |
| Solubility | Practically insoluble in water. |
| Vapor Pressure | 6.8X10-26 mm Hg at 25 °C /Estimated/ |
| LogP | 1.24 |
| Henry's Law Constant | Henry's Law constant = 5.8X10-32 atm-cu m/mol at 25 °C /Estimated/ |
| Optical Rotation | Specific optical rotation: -107 deg at 20 °C/D (9:1 chloroform/methanol) |
| Decomposition | When heated to decomposition it emits very toxic fumes of /sulfur oxides/. |
| Dissociation Constants | pKa = 10.13 |
| Collision Cross Section | 266.1 Ų [M+Na]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | Teniposide is a semisynthetic podophyllotoxin-derivative antineoplastic agent, which is structurally and pharmacologically related to etoposide. Teniposide differs structurally from etoposide by addition of a thenylidine ring on the glucopyranoside ring. |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 656.7 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 6 |
| Exact Mass | 656.15636224 g/mol |
| Monoisotopic Mass | 656.15636224 g/mol |
| Topological Polar Surface Area | 189 Ų |
| Heavy Atom Count | 46 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Teniposide is a semisynthetic derivative of podophyllotoxin that exhibits antitumor activity. Teniposide inhibits DNA synthesis by forming a complex with topoisomerase II and DNA. This complex induces breaks in double stranded DNA and prevents repair by topoisomerase II binding. Accumulated breaks in DNA prevent cells from entering into the mitotic phase of the cell cycle, and lead to cell death. Teniposide acts primarily in the G2 and S phases of the cycle.
