289499-45-2
Product Name:
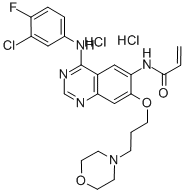
Canertinib dihydrochloride
Formula:
C24H27Cl3FN5O3
Synonyms:
Canertinib dihydrochloride;N-[4-(3-Chloro-4-fluorophenylamino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]acrylamide dihydrochloride;N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[3-(4-morpholinyl)propoxy]-6-quinazolinyl]-2-propenamide dihydrochloride;PD183805
Inquiry
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 558.9 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 557.116351 g/mol |
| Monoisotopic Mass | 557.116351 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 671 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Canertinib Dihydrochloride is the hydrochloride salt of an orally bio-available quinazoline with potential antineoplastic and radiosensitizing activities. Canertinib binds to the intracellular domains of epidermal growth factor receptor tyrosine kinases (ErbB family), irreversibly inhibiting their signal transduction functions and resulting in tumor cell apoptosis and suppression of tumor cell proliferation. This agent also acts as a radiosensitizing agent and displays synergistic activity with other chemotherapeutic agents.