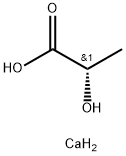
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder |
|---|---|
| Color/Form | White, crystalline powder |
| Melting Point | > 120 |
| Solubility | 48 g/L |
| Stability/Shelf Life | AQ SOLN ARE PRONE TO BECOME MOLDY. |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| pH | Between 6,0 and 8,0 (5 % solution) |
| Refractive Index | INDEX OF REFRACTION: 1.470 (ALPHA), 1.510 (GAMMA); ELONGATION: +, EXTINCTION PARALLEL |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 218.22 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 218.0103289 g/mol |
| Monoisotopic Mass | 218.0103289 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 53.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Calcium lactate is a salt that consists of two lactate anions for each calcium cation (Ca2+). It is prepared commercially by the neutralization of lactic acid with calcium carbonate or calcium hydroxide. Approved by the FDA as a direct food substance affirmed as generally recognized as safe, calcium lactate is used as a firming agent, flavoring agent, leavening agent, stabilizer, and thickener. Calcium lactate is also found in daily dietary supplements as a source of calcium. It is also available in various hydrate forms, where calcium lactate pentahydrate is the most common.