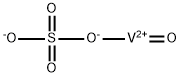
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Vanadyl sulfate appears as a blue crystalline solid. Very soluble in water. Denser than water. Contact may irritate skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. |
|---|---|
| Melting Point | MP: decomposes at 265 °C /Vanadyl sulfate dihydrate/ |
| Solubility | Solubility: In water, 467 g/L at 20 °C (pH 0.81-1.07) /Vanadyl sulfate pentahydrate/ |
| Density | Density: 2.06 at 20 °C, in comparison to water at 4 °C /Vanadyl sulfate pentahydrate/ |
| Stability/Shelf Life | Stable under recommended storage conditions. /Vanadium(IV) oxide sulfate hydrate/ |
| Decomposition | When heated to decomposition it emits toxic fumes of /vanadium and sulfur oxides/. |
| Other Experimental Properties | Blue crystals, soluble in water /Vanadyl sulfate dihydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 163.01 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 162.890602 g/mol |
| Monoisotopic Mass | 162.890602 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 64.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Vanadyl sulfate appears as a blue crystalline solid. Very soluble in water. Denser than water. Contact may irritate skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.