SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 578.5 g/mol |
|---|---|
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 3 |
| Exact Mass | 578.14242626 g/mol |
| Monoisotopic Mass | 578.14242626 g/mol |
| Topological Polar Surface Area | 221 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 925 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
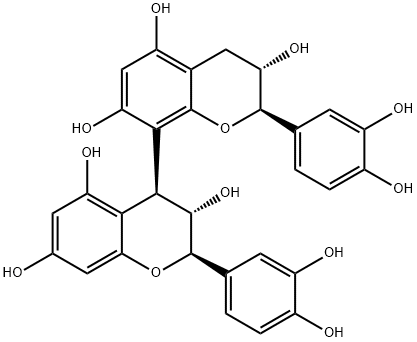
Procyanidin B3 is a proanthocyanidin consisting of two molecules of (+)-catechin joined by a bond between positions 4 and 8' in alpha-configuration. It can be found in red wine, in barley, in beer, in peach or in Jatropha macrantha, the Huanarpo Macho. It has a role as a metabolite, an antioxidant, an anti-inflammatory agent and an EC 2.3.1.48 (histone acetyltransferase) inhibitor. It is a hydroxyflavan, a proanthocyanidin, a biflavonoid and a polyphenol. It is functionally related to a (+)-catechin.