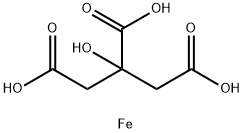
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White solid; [Hawley] |
|---|---|
| Melting Point | White microscopic rhombic, orthorhombic crystals; mp: decomposition at 350 °C in molecular hydrogen; soluble in ammonium hydroxide. /Monohydrate/ |
| Other Experimental Properties | Very stable to air oxidation. If water is removed by vacuum desiccation, the dehydrated ferrous salt rapidly oxidizes to a ferric salt. ...Very slightly colored powder or white crystals /Decahydrate/ |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
COMPUTED DESCRIPTORS
| Molecular Weight | 245.95 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 245.946288 g/mol |
| Monoisotopic Mass | 245.946288 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 234 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |