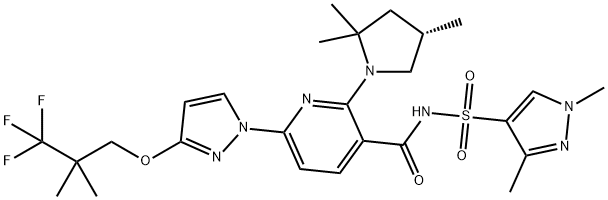
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | <1mg/mL |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 597.7 g/mol |
|---|---|
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 8 |
| Exact Mass | 597.23450825 g/mol |
| Monoisotopic Mass | 597.23450825 g/mol |
| Topological Polar Surface Area | 133 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Elexacaftor (previously VX-445) is a small molecule, next-generation corrector of the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It received FDA approval in October 2019 in combination with [tezacaftor] and [ivacaftor] as the combination product TrikaftaTM. Elexacaftor is considered a next-generation CFTR corrector as it possesses both a different structure and mechanism as compared to first generation correctors like tezacaftor. While dual corrector/potentiator combination therapy has proven useful in the treatment of a subset of CF patients, their use is typically limited to patients who are homozygous for the F508del-CFTR gene. Elexacaftor, along with [VX-659], was designed to fill the need for an efficacious CF therapy for patients who are heterozygous for F508del-CFTR and a gene that does not produce protein or produces proteins unresponsive to ivacaftor or tezacaftor. The triple combination product TrikaftaTM, manufactured by Vertex Pharmaceuticals, is the first product approved for the treatment of CF in individuals who are either homo- or heterozygous for the F508del-CFTR gene - this represents approximately 70-90% of all CF patients.