2179-25-1
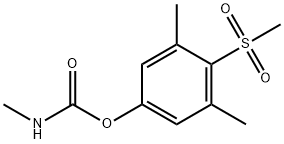
CHEMICAL AND PHYSICAL PROPERTIES
| Ionization Efficiency | Positive |
|---|---|
| Kovats Retention Index | 1886.9 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
COMPUTED DESCRIPTORS
| Molecular Weight | 257.31 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 257.07217913 g/mol |
| Monoisotopic Mass | 257.07217913 g/mol |
| Topological Polar Surface Area | 80.8 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 361 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Methiocarb sulfone is a carbamate pesticide. Carbamate pesticides are derived from carbamic acid and kill insects in a similar fashion as organophosphate insecticides. They are widely used in homes, gardens and agriculture. The first carbamate, carbaryl, was introduced in 1956 and more of it has been used throughout the world than all other carbamates combined. Because of carbaryl's relatively low mammalian oral and dermal toxicity and broad control spectrum, it has had wide use in lawn and garden settings. Most of the carbamates are extremely toxic to Hymenoptera, and precautions must be taken to avoid exposure to foraging bees or parasitic wasps. Some of the carbamates are translocated within plants, making them an effective systemic treatment. (L795)