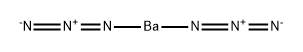CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Barium azide, wetted with not less than 50% water appears as a slurry of white crystals. When dry, a high explosive that easily ignited and quick to burn vigorously. Wetting reduces sensitivity to shock and heat. Generates toxic oxides of nitrogen when burned. |
|---|---|
| Chemical Classes | Other Uses -> Explosives |
COMPUTED DESCRIPTORS
| Molecular Weight | 221.37 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 221.923691 g/mol |
| Monoisotopic Mass | 221.923691 g/mol |
| Topological Polar Surface Area | 6 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Barium azide, wetted with not less than 50% water appears as a slurry of white crystals. When dry, a high explosive that easily ignited and quick to burn vigorously. Wetting reduces sensitivity to shock and heat. Generates toxic oxides of nitrogen when burned.