CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Colorless, crystalline powder |
|---|---|
| Odor | Odorless |
| Melting Point | 200 °C (decomposes) |
| Solubility | In water, 44 g/L at 20 °C (pH 9) |
| Vapor Pressure | VP: 1X10-10 mm Hg at 20 °C |
| LogP | log Kow: -0.89 (pH 4); -1.85 (pH 7); -1.89 (pH 9) |
| Decomposition | When heated to decomposition it emits toxic vapors of /nitrogen oxides, sulfur oxides, and fluoride/. |
| Dissociation Constants | pKa = 1.9 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
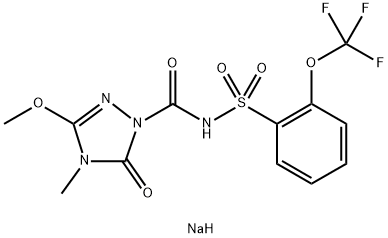
| Molecular Weight | 418.28 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 418.01708399 g/mol |
| Monoisotopic Mass | 418.01708399 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 711 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Flucarbazone-sodium is an organic sodium salt resulting from the formal reaction of the sulfonamide amino group of flucarbazone with 1 mol eq. of sodium hydride. An acetolactate synthase inhibitor, it is used as a herbicide to control grass weeds in cereal crops. Not approved for use within the European Union. It has a role as an EC 2.2.1.6 (acetolactate synthase) inhibitor, a herbicide and an agrochemical. It contains a flucarbazone(1-).