CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | 1.4 mg/mL at acidic PH (2) and |
|---|
COMPUTED DESCRIPTORS
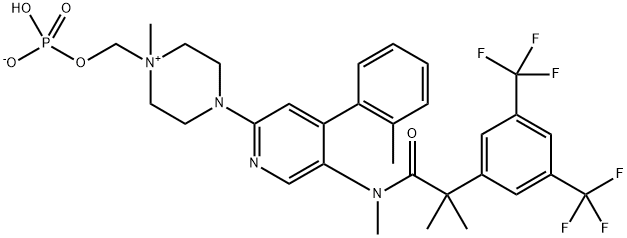
| Molecular Weight | 688.6 g/mol |
|---|---|
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 8 |
| Exact Mass | 688.22492620 g/mol |
| Monoisotopic Mass | 688.22492620 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 1100 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
In April 2018, the U.S. Food and Drug Administration (FDA) and the Swiss company Helsinn approved the intravenous formulation of AKYNZEO® (NEPA, a fixed antiemetic combination of fosnetupitant, 235mg, and palonosetron, 0.25mg) as an alternative treatment option for patients experiencing chemotherapy-induced nausea and vomiting. Fosnetupitant is the pro-drug form of netupitant. Generally, 25% to 30% of patients with a diagnosis of cancer receive chemotherapy as a treatment modality and 70% to 80% of these patients undergoing chemotherapy treatment may experience nausea and vomiting as major side effects. Considered one of the most distressing side effects of chemotherapy, nausea and vomiting has an immense impact on the quality of life of patients receiving certain antineoplastic therapies.