16674-78-5
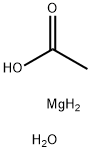
Product Name:
Magnesium acetate tetrahydrate
Formula:
C2H8MgO3
Synonyms:
Acetic acid magnesium salt;Magnesium acetate tetrahydrate
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless or white deliquescent solid; [Merck Index] White hygroscopic solid with an odor like vinegar; [Acros Organics MSDS] |
|---|---|
| Boiling Point | 117.1 °C at 760 mmHg |
| Melting Point | 80 |
| Solubility | Soluble |
| Chemical Classes | Metals -> Organic Acids, Metal Salts |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 214.45 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 214.0539091 g/mol |
| Monoisotopic Mass | 214.0539091 g/mol |
| Topological Polar Surface Area | 84.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 7 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Magnesium acetate tetrahydrate is a hydrated form of anhydrous magnesium acetate salt with the chemical formula of Mg(CH3COO)2 • 4H2O. As a salt form of magnesium, magnesium acetate is one of the bioavailable forms of magnesium and forms a very water soluble compound. Magnesium is an essential element and second most abundant cation in the body that plays a key role in maintaining normal cellular function such as production of ATP and efficient enzyme activity. Magnesium acetate tetrahydrate can be used as an electrolyte supplementation or a reagent in molecular biology experiments.
