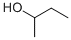| Physical Description |
Sec-butyl alcohol appears as a clear colorless liquid with an alcohol odor. Flash point below 0 °F. Less dense than water. Vapors heavier than air. Soluble in water. Moderately irritates the eyes and skin. Prolonged and repeated contact may cause defatting and drying of the skin. Vapors may irritate the nose, throat and respiratory tract. May be harmful by ingestion. |
| Color/Form |
Colorless liquid |
| Odor |
HAS A STRONG VINOUS ODOR |
| Boiling Point |
211 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point |
-175 °F (NIOSH, 2023) |
| Flash Point |
75 °F (NIOSH, 2023) |
| Solubility |
16 % (NIOSH, 2023) |
| Density |
0.81 (NIOSH, 2023) - Less dense than water; will float |
| Vapor Density |
2.6 (Air= 1) |
| Vapor Pressure |
12 mmHg (NIOSH, 2023) |
| LogP |
log Kow= 0.61 |
| Henry's Law Constant |
Henry's Law constant = 9.06X10-6 atm-cu m/mol @ 25 °C |
| Stability/Shelf Life |
FEASIBLE AUTO OXIDATION BY ATMOSPHERIC OXYGEN TO FORM PEROXIDES & PEROXY CMPD |
| Autoignition Temperature |
763 °F (406 °C) (IN AIR); 711 °F (377 °C) (IN OXYGEN) |
| Decomposition |
When heated to decomposition it emits acrid smoke & fumes. |
| Viscosity |
4.21 cP at 15 °C |
| Corrosivity |
sec-Butyl alcohol will attack some forms of plastics, rubber, and coatings. |
| Heat of Combustion |
-15,500 BTU/lb= -8600 cal/g= -360x10+5 J/kg |
| Heat of Vaporization |
49.72 kJ/mol @ 25 °C |
| Surface Tension |
23.0 dynes/cm= 0.023 N/m at 20 °C |
| Ionization Potential |
10.10 eV |
| Odor Threshold |
Odor Threshold Low: 0.12 [mmHg] Odor Threshold High: 13.8 [mmHg] Detection odor threshold from AIHA (mean = 3.2 ppm) |
| Dielectric Constant |
Dielectric constant: 16.6 at 25 °C; dipole moment: 1.66 dehye (in benzene at 30 °C) |
| Refractive Index |
Index of refraction: 1.3978 @ 20 °C/D |
| Dissociation Constants |
pKa = 17.6 |
| Relative Evaporation Rate |
1.3 (butyl acetate= 1) |
| Kovats Retention Index |
600.6 602.2 565 585 586 565 585 587 594 576 577 605 577 605 577 605 585 586 570 584 586 586 590 593 595 586 586 586 586 591 582 588.4 589 613 587 587.64 561.1 596 585 585 624 591 596 603 586 628 596.4 |
| Other Experimental Properties |
IT EXISTS AS D, L, & DL ISOMERS |
| Chemical Classes |
Solvents -> Alcohols (<C12) |