156-57-0
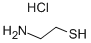
Product Name:
Cysteamine hydrochloride
Formula:
C2H8ClNS
Synonyms:
β-Mercapto-ethylamine hydrochloride;2-Amino-ethane-thiol hydrochloride;2-Aminoethanethiolhydrochloride, Cysteamine hydrochloride, 2-Mercaptoethylamine hydrochloride;2-Mercapto-ethyl-amine hydrochloride;Cysteaminium chloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] White hygroscopic crystals; [Alfa Aesar MSDS] |
|---|---|
| Solubility | >17 [ug/mL] (The mean of the results at pH 7.4) |
| Chemical Classes | Biological Agents -> Amino Acids and Derivatives |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 113.61 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 113.0065981 g/mol |
| Monoisotopic Mass | 113.0065981 g/mol |
| Topological Polar Surface Area | 27 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 10 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
A mercaptoethylamine compound that is endogenously derived from the COENZYME A degradative pathway. The fact that cysteamine is readily transported into LYSOSOMES where it reacts with CYSTINE to form cysteine-cysteamine disulfide and CYSTEINE has led to its use in CYSTINE DEPLETING AGENTS for the treatment of CYSTINOSIS.
