CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | White to light tan powder |
| Solubility | Practically insoluble |
| Vapor Pressure | 1.1X10-27 mm Hg @ 25 °C /Estimated/ |
| LogP | 3.9 |
| Henry's Law Constant | Henry's Law constant = 2.7X10-33 atm-cu m/mol @ 25 °C /Estimated/ |
| Other Experimental Properties | Hydroxyl radical reaction rate constant = 9.7X10-11 cu cm/molec-sec @ 25 °C /Estimated/ |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 720.9 g/mol |
|---|---|
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 18 |
| Exact Mass | 720.31276100 g/mol |
| Monoisotopic Mass | 720.31276100 g/mol |
| Topological Polar Surface Area | 202 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 1040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
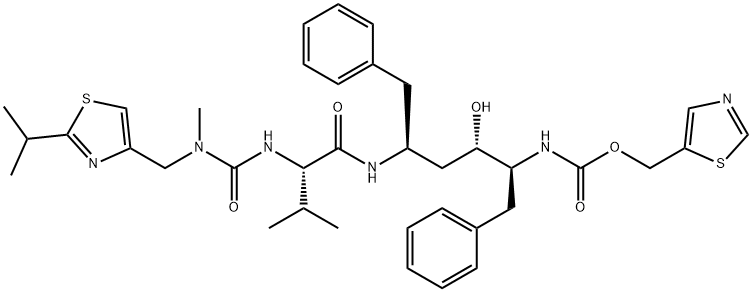
Ritonavir is an L-valine derivative in which the alpha-amino group is acylated by a [(2-isopropyl-1,3-thiazol-4-yl)methyl]methylcarbamoyl group and a hydrogen of the carboxamide amino group is replaced by a (2R,4S,5S)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl group. As a CYP3A inhibitor and antiretroviral drug from the protease inhibitor class, it is used to treat HIV infection and AIDS, often in fixed-dose combination with another protease inhibitor, lopinavir. Additionally, ritonavir is used in combination with dasabuvir sodium hydrate, ombitasvir, and paritaprevir (under the trade name Viekira Pak) for the treatment of chronic hepatitis C virus genotype 1 infection and cirrhosis of the liver. Ritonavir serves as an antiviral drug, HIV protease inhibitor, environmental contaminant, and xenobiotic. It belongs to the classes of 1,3-thiazoles, L-valine derivatives, carbamate esters, ureas, and carboxamides.