14882-18-9
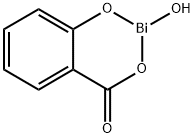
Product Name:
BISMUTH SUBSALICYLATE
Formula:
C7H5BiO4
Synonyms:
Bismuth oxysalicylate;Bismuth subsalicylate;Bismuth(III) salicylate basic
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Bismuth subsalicylate appears as white crystalline powder or fluffy white solid. (NTP, 1992) |
|---|---|
| Color/Form | MICROSCOPIC PRISMS |
| Odor | ODORLESS |
| Taste | TASTELESS |
| Melting Point | >350 |
| Solubility | Insoluble (<1 mg/ml at 71.1 °F) (NTP, 1992) |
| Vapor Pressure | 0.00000607 [mmHg] |
| Stability/Shelf Life | STABLE IN AIR BUT AFFECTED BY LIGHT |
| Other Experimental Properties | DECOMP BY BOILING WATER, ALKALIES INTO MORE BASIC SALT |
| Chemical Classes | Metals -> Metals, Organic Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
COMPUTED DESCRIPTORS
| Molecular Weight | 363.10 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 363.00701 g/mol |
| Monoisotopic Mass | 363.00701 g/mol |
| Topological Polar Surface Area | 36.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 173 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Bismuth subsalicylate appears as white crystalline powder or fluffy white solid. (NTP, 1992)