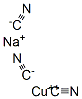CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Sodium cuprocyanide, solid appears as a white powder. Decomposes at 212 °F. Toxic by inhalation, ingestion and skin absorption through open wounds. Reacts with acids to evolve hydrogen cyanide, which is a highly toxic and flammable. Used for preparing and maintaining copper plating baths. |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 187.58 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 186.918358 g/mol |
| Monoisotopic Mass | 186.918358 g/mol |
| Topological Polar Surface Area | 71.4 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 53.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 6 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Sodium cuprocyanide, solid appears as a white powder. Decomposes at 212 °F. Toxic by inhalation, ingestion and skin absorption through open wounds. Reacts with acids to evolve hydrogen cyanide, which is a highly toxic and flammable. Used for preparing and maintaining copper plating baths.