142-62-1
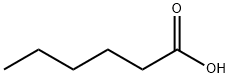
Product Name:
Hexanoic acid
Formula:
C6H12O2
Synonyms:
Acid C6;Caproic acid;Hexanoic acid
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Caproic acid appears as a white crystalline solid or colorless to light yellow solution with an unpleasant odor. Insoluble to slightly soluble in water and less dense than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make perfumes. |
|---|---|
| Color/Form | Oily liquid |
| Odor | Characteristic goat-like odor |
| Boiling Point | 396 to 397 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 27 °F (NTP, 1992) |
| Flash Point | 220 °F (NTP, 1992) |
| Solubility | 5 to 10 mg/mL at 72 °F (NTP, 1992) |
| Density | 0.927 (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 4.01 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.2 mmHg at 68 °F ; 1 mmHg at 158 °F; 20 mmHg at 233.2 °F (NTP, 1992) |
| LogP | log Kow = 1.92 |
| Henry's Law Constant | Henry's Law constant = 7.58X10-7 atm cu m/mol at 25 °C |
| Autoignition Temperature | 716 °F (NTP, 1992) |
| Decomposition | When heated to decomposition it emits acrid smoke and fumes. |
| Viscosity | 3.23 mPa.s at 20 °C |
| Heat of Combustion | -3,492.4 KJ/mol (liquid) |
| Surface Tension | 23.4 mN/m at 70 °C |
| Odor Threshold | Odor Threshold Low: 6000.0 [mmHg] Odor threshold (100% recognition) from CHEMINFO |
| Refractive Index | Index of refraction: 1.4170 at 20 C/D |
| Dissociation Constants | pKa = 4.88 |
| Collision Cross Section | 128.54 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Kovats Retention Index | 973 962 988 951 956 988 983 996 992 982 948 992 967 1010 975 981 968 957 950 974 999 958 1007 978 1006 1011 1000 1013 1005 1005 983 1008 966 995 965 1007 1005 1005 975 964 981 981 975 961 970 997 |
| Other Experimental Properties | Heat of formation: -584.0 KJ/mol; Specific heat 2.33 J/g |
| Chemical Classes | Other Classes -> Organic Acids |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 116.16 g/mol |
|---|---|
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 116.083729621 g/mol |
| Monoisotopic Mass | 116.083729621 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 68.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Caproic acid appears as a white crystalline solid or colorless to light yellow solution with an unpleasant odor. Insoluble to slightly soluble in water and less dense than water. Contact may severely irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make perfumes.
RELATED SUPPLIERS
Madhu Chemicals
Mumbai
product:Caproic Acid /Hexanoic Acid, CAS Number: 142-62-1, Packaging Size: 180 Kgs
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
Gujarat
product:N-Caproic Acid (142-62-1)
