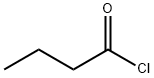
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Butyryl chloride appears as a clear colorless liquid with a sharp odor. Slightly denser than water. Flash point near 20 °F. Severely irritates skin, eyes, and mucous membranes. Used to make other chemicals. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Pungent acid chloride odor |
| Boiling Point | 216 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -128 °F (NTP, 1992) |
| Flash Point | 71 °F (NTP, 1992) |
| Solubility | Reaction (NTP, 1992) |
| Density | 1.0277 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Density | 3.67 (vapor specific gravity) |
| Vapor Pressure | 18.8 mmHg at 28 °F ; 41.7 mmHg at 77 °F; 95.2 mmHg at 108 °F (NTP, 1992) |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Dissolves slowly with decomposition in water, alcohol. |
| Corrosivity | Corrosive to metals |
| Refractive Index | Index of refraction: 1.4121 at 20 °C |
| Kovats Retention Index | 705 |
| Other Experimental Properties | Fumes in air. Decomposes exothermically in water to give corrosive hydrochloric acid. Based on a scenario where the chemical is spilled into an excess of water (at least 5 fold excess of water), half of the maximum theoretical yield of Hydrogen Chloride gas will be created in 0.47 minutes. |
| Chemical Classes | Toxic Gases & Vapors -> Other Toxic Gases & Vapors |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 106.55 g/mol |
|---|---|
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 106.0185425 g/mol |
| Monoisotopic Mass | 106.0185425 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 51.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Butyryl chloride appears as a clear colorless liquid with a sharp odor. Slightly denser than water. Flash point near 20 °F. Severely irritates skin, eyes, and mucous membranes. Used to make other chemicals.