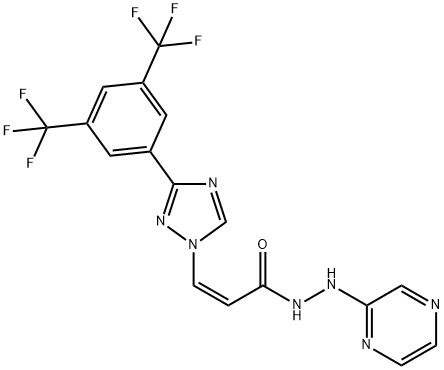
CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | 20mg/mL |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 443.3 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 5 |
| Exact Mass | 443.09292697 g/mol |
| Monoisotopic Mass | 443.09292697 g/mol |
| Topological Polar Surface Area | 97.6 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 621 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Selinexor is a first-in-class selective inhibitor of nuclear transport (SINE) compound. Selinexor, in combination with [bortezomib] and [dexamethasone], is currently approved for the treatment of multiple myeloma, a type of cancer formed from antibody-producing plasma cells. This condition is typically treated with high dose [bortezomib] and [dexamethasone] chemotherapy followed by an autologous stem-cell transplant. Other chemotherapies for multiple myeloma include [lenalidomide] and [dexamethasone], [thalidomide], and may include [melphalan] if the patient is not eligible for transplant. Selinexor was also granted accelerated approval for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) that have gone through at least 2 lines of systemic therapy. The FDA approved Selinexor in June 2019. The use of selinexor in combination with [bortezomib] and [dexamethasone] was approved by Health Canada in June 2022 for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.