CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White odorless solid; [HSDB] Powder; [MSDSonline] |
|---|---|
| Color/Form | White crystals |
| Odor | Odorless |
| Melting Point | 98.9 °C |
| Solubility | In water, 4.25X10+3 mg/L at 25 °C |
| Density | 1.330 g/cu cm at 20 °C |
| Vapor Pressure | 0.000044 [mmHg] |
| LogP | log Kow = 0.80 at 25 °C |
| Henry's Law Constant | Henry's Law constant = 6.92X10-8 atm-cu m/mol at 25 °C /Estimated/ |
| Ionization Efficiency | Positive |
| Dissociation Constants | pKa = 0.7 |
| Collision Cross Section | 150.24 Ų [M+H]+ [CCS Type: TW] |
| Kovats Retention Index | 2452 2458 2435.8 |
| Chemical Classes | Pesticides -> Insecticides, Neonicotinoid |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H330:Acute toxicity,inhalation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P284:Wear respiratory protection. P310:Immediately call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 222.67 g/mol |
|---|---|
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 222.0672241 g/mol |
| Monoisotopic Mass | 222.0672241 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 280 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
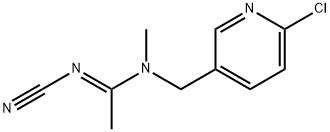
Acetamiprid is a carboxamidine that is acetamidine in which the amino hydrogens are substituted by a (6-chloropyridin-3-yl)methyl and a methyl group while the hydrogen attached to the imino nitrogen is replaced by a cyano group. It has a role as a neonicotinoid insectide, an environmental contaminant and a xenobiotic. It is a monochloropyridine, a nitrile and a carboxamidine. It is functionally related to a 2-chloropyridine.