133-32-4
Product Name:
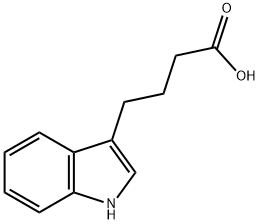
Indole-3-butyric acid
Formula:
C12H13NO2
Synonyms:
4-(3-Indolyl)butanoic acid;4-(3-Indolyl)butyric acid;IBA
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White or slightly yellow solid; [Merck Index] Off-white powder; [MSDSonline] |
|---|---|
| Color/Form | White to slightly yellow crystals |
| Odor | Essentially odorless |
| Melting Point | 124.5 °C |
| Solubility | 250 mg/L (at 20 °C) |
| Vapor Pressure | 0.00000117 [mmHg] |
| LogP | 2.3 |
| Henry's Law Constant | Henry's Law constant: 1.3X10-11 atm cu m/mol@ 25 °C /Estimated/ |
| Stability/Shelf Life | Very stable in neutral, acidic and alkaline media. |
| Dissociation Constants | pKa = 4.7 /Estimated/ |
| Kovats Retention Index | 1885 |
| Other Experimental Properties | Hydroxyl radical reaction rate constant: 2.0X10-10 cu cm/molecule-sec @ 25 °C /Estimated/ |
| Chemical Classes | Pesticides -> Plant Growth Regulators |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 203.24 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 203.094628657 g/mol |
| Monoisotopic Mass | 203.094628657 g/mol |
| Topological Polar Surface Area | 53.1 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 230 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Indole-3-butyric acid is a substance that is closely related in structure and function to a natural growth regulator found in plants. Indole-3-butyric acid is used on many crops and ornamentals to promote growth and development of roots, flowers and fruits, and to increase crop yields. Growers find it more effective and efficient than its natural counterpart because plants cannot break it down as quickly. No harm to humans or the environment is expected to result from use of indole-3-butyric acid.
RELATED SUPPLIERS
All suppliers(13)
