1314-13-2
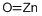
Product Name:
Zinc oxide
Formula:
OZn
Synonyms:
NanoSunguard in butyl acetate;Zinc oxide;ZN526010
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
PRODUCT INTRODUCTION
Description
Zinc oxide is a versatile chemical used as a sunscreen in cosmetics; a catalyst in the chemical industry; a corrosion inhibitor and mildew inhibitor in paints; a filler in pigments; and a skin protectant in medicine.
