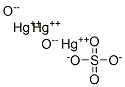CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Mercuric subsulfate, [solid] appears as a lemon-yellow powder or bright yellow scaly material. Very slightly soluble in water and denser than water. Hence sinks in water. Toxic by inhalation and by ingestion. |
|---|---|
| Color/Form | Lemon-yellow powder |
| Odor | Odorless |
| Solubility | SOLUBILITY IN WATER @ 16 °C: 0.003 G/100 CC; SLIGHTLY SOL IN HOT WATER; INSOL IN ALCOHOL |
| Density | 6.44 |
| Vapor Density | 25.2 (Air= 1) |
| Other Experimental Properties | Proved to be mercury oxonium sulfate in x-ray studies of its structure. Polymeric oxonium cations (Hg3O2)x form 2-dimensional infinite layers. The sulfate ions lie inside loops of wide-meshed cation lattice. |
COMPUTED DESCRIPTORS
| Molecular Weight | 729.84 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 728.84881 g/mol |
| Monoisotopic Mass | 733.85349 g/mol |
| Topological Polar Surface Area | 90.6 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 6 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Mercuric subsulfate, [solid] appears as a lemon-yellow powder or bright yellow scaly material. Very slightly soluble in water and denser than water. Hence sinks in water. Toxic by inhalation and by ingestion.