1305-62-0
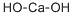
Product Name:
Calcium hydroxide
Formula:
CaH2O2
Synonyms:
Calcium dihydroxide;Calcium hydroxide;Carboxide
Inquiry
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
PRODUCT INTRODUCTION
Description
Calcium hydroxide (chemical formula Ca(OH)₂) is a soft white powder produced by mixing calcium oxide with water and widely used as a raw material in the chemical industry. As an ionic compound, it dissociates into calcium and hydroxide ions in aqueous solutions and during electrolysis. It serves as the main component in masonry, gypsum, and cement mortars; is added to food to prevent excessive acidity; and is used in the manufacture of insecticides, paints, waterproof materials, as well as an additive in oils and lubricants.
RELATED SUPPLIERS
All suppliers(17)
